
Open-Source Internship opportunity by OpenGenus for programmers. Apply now.
In this article at OpenGenus, we have explored the concept of Infrared Spectroscopy along with related concepts like Hooke's law and others.
Table of contents:
- Principle
- Infrared region is divided into following regions
- Infrared light can produce 3 types of controlled vibrations
- Symmetrical stretching
- Asymmetrical stretching
- Bending
- Selection route for IR Spectroscopy
- Regions of IR spectra
- Vibrational frequency (Hooke's Law)
- Difference between IR and UV spectrum
Principle
- "Vibrational(IR) spectroscopy involves the transitions between the vibrational energy levels of a molecule on the absorption of radiations falling in the spectral range of 500/cm- 4000/cm(infrared region)."
- Vibrational spectra appear as vibrational-rotational bands as a single vibrational energy change is accompanied by a large number of rotational energy changes.
Infrared region is divided into following regions:
- Near-infrared region- Part of IR spectrum closest to visible light(400nm-700nm)
- Far-infrared region- Part of IR spectrum closest to microwave region(3mm-30cm)
- Mid-infrared region- Part of IR spectrum between near and far infrared regions
Thus, wavelength of infrared waves is longer than visible and shorter than microwaves.
Infrared light can produce 3 types of controlled vibrations:
- Stretching
- Symmetrical
- Asymmetrical
- Bending
Symmetrical stretching
- Stretching and compression of bonds in same direction/ symmetrical or alternate manner.
- Bond axis remains fixed.
- Only bond length changes.
Asymmetrical stretching
- Bonds are stretched in opposite directions or in simple words one bond is stretched and another is compressed.
- Bond axis remains fixed.
- Only bond length changes.
Bending
- Bond angle changes but bond length remains constant
- Takes place within the plane and out of plane
- In plane bending vibrations
- Rocking: Involve movement of atoms in same direction
- Scissoring: Two atoms are joined to a central atom and move towards or away from each other with change in bond angle
- Out of plane bending vibrations
- Wagging: Both atoms move up and below the plane with respect to central atom.
- Twisting: Movement of one atom up and the other atom down the plane with respect to the central atom.
Selection route for IR Spectroscopy
- Vibration must cause a change in dipole moment of the molecule.
- If a molecule is polar, then only it can show IR spectra and the molecule is IR active.
- Example:HCl, CO
- If a molecule is non-polar, then it cannot show IR spectra and the molecule is IR inactive.
- Example: H2, N2, O2, Cl2
- Exception: CO2, being non-polar, is IR- active
- Reason: Due to asymmetric stretching of CO2, a change occurs in dipole moment and it becomes IR- active.
Regions of IR spectra
- Functional group region:
- High frequency region, between 4000/cm-1300/cm
- Characteristic stretching frequencies for important functional groups such as CO, OH and NH occur in this region.
- Finger print region:
- Middle frequency region, between 1300/cm- 900/cm.
- Complex absorptions occur due to combinations of interacting vibrational modes, providing unique fingerprint to each molecule.
- Pattern of bands are dependent on the structure of molecule.
- This pattern is highly sensitive and even to minor changes in structure and stereochemistry.
- Low- frequency region
- Region between 900/cm-650/cm
- Provides general classification of molecules from the pattern of absorption, such as substitution patterns on benzene ring.
- Absence of absorptions provide a good evidence for absence of an aromatic compound.
Vibrational frequency (Hooke's Law)
- Hooke's Law helps to determine stretching frequency.
- Vibrational frequency= 1/(2πc)xsqrroot(k/u)
c= velocity of light
k= force constant
u= reduced mass
u=m1m2/m1+m2
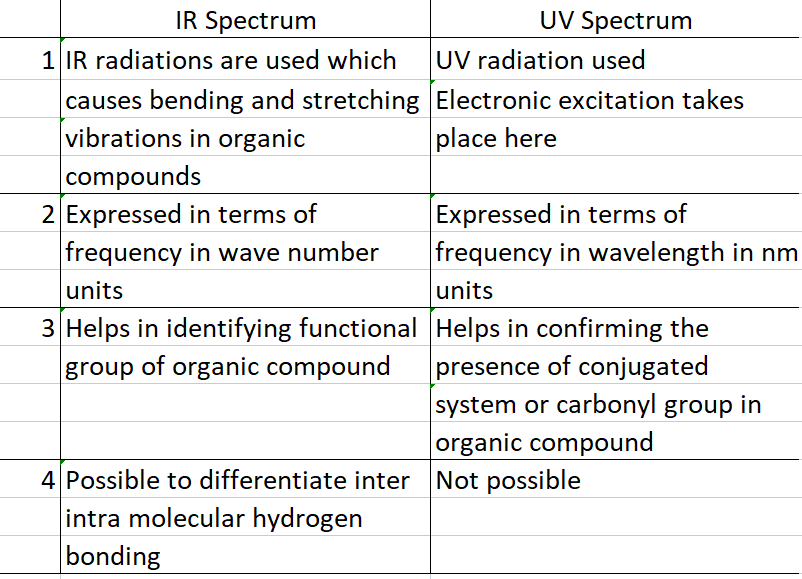
Difference between IR and UV spectrum
With this article at OpenGenus, you must have the complete idea of Infrared Spectroscopy.
